Lecture 4 (Day 2):
Light as Information
Models • Spectral Fingerprints • Telescopes
February 5, 2026
Learning Objectives
By the end of today, you will be able to:
- Use limiting-case reasoning to interpret the shape of a blackbody spectrum (Rayleigh–Jeans vs Wien tail)
- Explain why atoms produce absorption and emission lines and identify the three spectrum types (Kirchhoff)
Learning Objectives (continued)
- Describe how a real stellar spectrum combines continuum + lines
- Apply the two key telescope scalings: collecting area \(\propto D^2\) and resolution \(\propto \lambda D^{-1}\)
- Apply the Doppler equation to connect line shifts to radial velocity
Same object.
Different wavelength.
Different story.
Today’s Game Plan
We finish the “radiation foundation” in three moves:
- Why the blackbody curve has simple limits (so we can use it)
- How atoms add spectral lines (so we can infer composition)
- How telescopes change what we can measure (photons + resolution)
Quick Recall (Day 1)
Fill in the blanks:
- Waves: \(c = \lambda\nu\)
- Photons: \(E = hc\lambda^{-1}\)
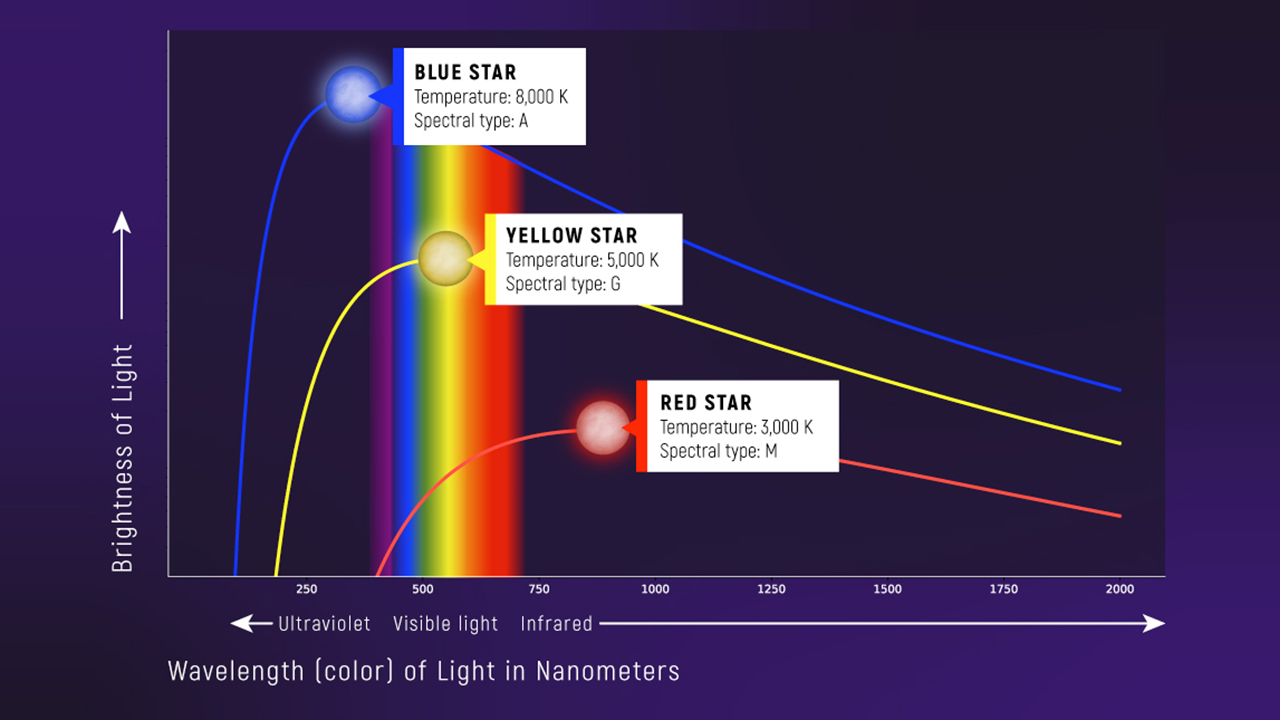
- Thermal peak: \(\lambda_{\mathrm{peak}} \propto T^{-1}\)
🧠 Quiz: Temperature from Color
A thermal spectrum peaks at a shorter wavelength. The object is…
- hotter
- cooler
- the same temperature
- necessarily younger
Planck + Wien (Thermal Equilibrium)
Thermal equilibrium means energy absorbed equals energy emitted, so the temperature is stable and the spectrum depends only on \(T\).
Planck function (blackbody spectrum)
What it predicts
Spectral radiance \(B_\lambda\) of a blackbody as a function of wavelength.
What it depends on
Wavelength \(\lambda\) and temperature \(T\), with constants \(h\), \(c\), and \(k_B\).
What it's saying
Two factors compete: the \(\lambda^{-5}\) term pushes short wavelengths up, while the exponential term suppresses them. Because \(h\nu\) and \(k_B T\) are both energies, the ratio \(h\nu/k_B T\) (or \(hc/\lambda k_B T\)) is dimensionless and sets the regime: \(h\nu \ll k_B T\) → Rayleigh–Jeans power law; \(h\nu \gg k_B T\) → Wien tail.
Assumptions
- Object radiates as an ideal blackbody in thermal equilibrium
- Spectrum expressed per unit wavelength interval
See: the equation
Wien’s law anchors the peak wavelength:
\[ \lambda_{\text{peak}} = b T^{-1} \tag{1}\]
Part 1: The Planck Curve Has “Handlebars”
Limiting cases make scary equations usable.
The Story Behind the Curve
Classically, you might expect short wavelengths to dominate because:
- shorter \(\lambda\) often means higher energy per photon
- power laws like \(\lambda^{-4}\) rise fast as \(\lambda\) shrinks
The Ultraviolet Catastrophe (What Went Wrong)
Classical physics predicts a spectrum that blows up at short wavelength.
That would mean infinite energy in the ultraviolet.
Reality: the spectrum peaks and then drops.
Planck’s Quantum Move
Energy is emitted/absorbed in chunks:
\[E = h\nu\]
- high frequency (short \(\lambda\)) photons cost more energy per photon
- at a given \(T\), very high-energy photons are rare
The Planck Function (Recognize It)
You don’t memorize it — you recognize it and read its structure:
\[ B_\lambda(T)=\frac{2hc^2}{\lambda^5}\cdot\frac{1}{e^{hc/(\lambda k_B T)}-1} \tag{2}\]
- \(B_\lambda\): spectral radiance (brightness per wavelength interval)
- \(\lambda\): wavelength, \(T\): temperature
- Units: erg·s\(^{-1}\)·cm\(^{-2}\)·sr\(^{-1}\)·cm\(^{-1}\)
How to Read \(B_\lambda(T)\) (Study Version)
Read it like a sentence:
Planck function (blackbody spectrum)
What it predicts
Spectral radiance \(B_\lambda\) of a blackbody as a function of wavelength.
What it depends on
Wavelength \(\lambda\) and temperature \(T\), with constants \(h\), \(c\), and \(k_B\).
What it's saying
Two factors compete: the \(\lambda^{-5}\) term pushes short wavelengths up, while the exponential term suppresses them. Because \(h\nu\) and \(k_B T\) are both energies, the ratio \(h\nu/k_B T\) (or \(hc/\lambda k_B T\)) is dimensionless and sets the regime: \(h\nu \ll k_B T\) → Rayleigh–Jeans power law; \(h\nu \gg k_B T\) → Wien tail.
Assumptions
- Object radiates as an ideal blackbody in thermal equilibrium
- Spectrum expressed per unit wavelength interval
See: the equation
How to Read the Curve (Where the Limits Live)
Use the curve as your map:
- Right side (long \(\lambda\)): \(h\nu \ll k_B T\) → Rayleigh–Jeans power law
- Left side (short \(\lambda\)): \(h\nu \gg k_B T\) → Wien exponential drop

You Don’t Memorize Planck — You Read It
The blackbody curve has two key regimes:
- Long-wavelength tail (Rayleigh–Jeans)
- Short-wavelength tail (Wien)
Those regimes let you predict behavior without re-deriving the whole function.

What the Planck Function Does
Even without memorizing it, recognize the structure:
\[ B_\lambda(T)=\frac{2hc^2}{\lambda^5}\cdot\frac{1}{e^{hc/(\lambda k_B T)}-1} \tag{3}\]
- power‑law prefactor (\(2hc^2/\lambda^5\)) rises steeply toward short \(\lambda\)
- exponential term (\(e^{hc/\lambda k_B T}\)) suppresses short \(\lambda\)
- because \(h\nu\) and \(k_B T\) are both energies, \(h\nu/k_B T\) is the dimensionless “regime knob”

Long Wavelengths (Rayleigh–Jeans Limit)
At long wavelengths, the spectrum behaves like:
\[B_\lambda \propto T\lambda^{-4}\]
- at fixed \(\lambda\): hotter → brighter (linear in \(T\))
- at fixed \(T\): shorter \(\lambda\) → much brighter (\(\lambda^{-4}\) is steep)
Where Does Rayleigh–Jeans Apply?
Rayleigh–Jeans is a long-wavelength approximation.
- Condition: \(h\nu \ll k_B T\)
- On the curve, it’s the right side of the peak (long \(\lambda\)).

🧠 Predict (Rayleigh–Jeans)
Rayleigh–Jeans: \(B_\lambda \propto T\lambda^{-4}\) (long \(\lambda\), \(h\nu \ll k_B T\)).
At fixed temperature, if wavelength doubles, \(B_\lambda\) changes by…
- \(\times 2\)
- \(\times 4\)
- \(\times 8\)
- \(\div 16\)
Short Wavelengths (Wien Tail)
At short wavelengths, the spectrum drops off exponentially:
\[B_\lambda \approx \left(\text{power law}\right)\times e^{-hc/\lambda k_B T}\]
Interpretation: very high-energy photons are “expensive” thermally.
Where Does the Wien Tail Apply?
The Wien tail is the short-wavelength side of the curve.
- Condition: \(h\nu \gg k_B T\)
- On the curve, it’s the left side of the peak (short \(\lambda\)).
That’s why hot objects can emit UV, but not “infinite UV.”

What Limiting Cases Buy Us
Instead of memorizing a complicated formula, we can answer questions like:
- “Which side of the peak am I on?”
- “Does intensity fall as a power law or exponentially?”
- “What changes faster: with \(T\) or with \(\lambda\)?”
Example: The Cosmic Microwave Background
The CMB is an almost perfect blackbody:
- temperature \(\approx 2.725\ \mathrm{K}\)
- peak is in the microwave
It’s “thermal radiation from the early universe,” stretched and cooled by cosmic expansion.
A tiny part of old analog TV “snow” was this same microwave background — a faint hiss from the early universe.
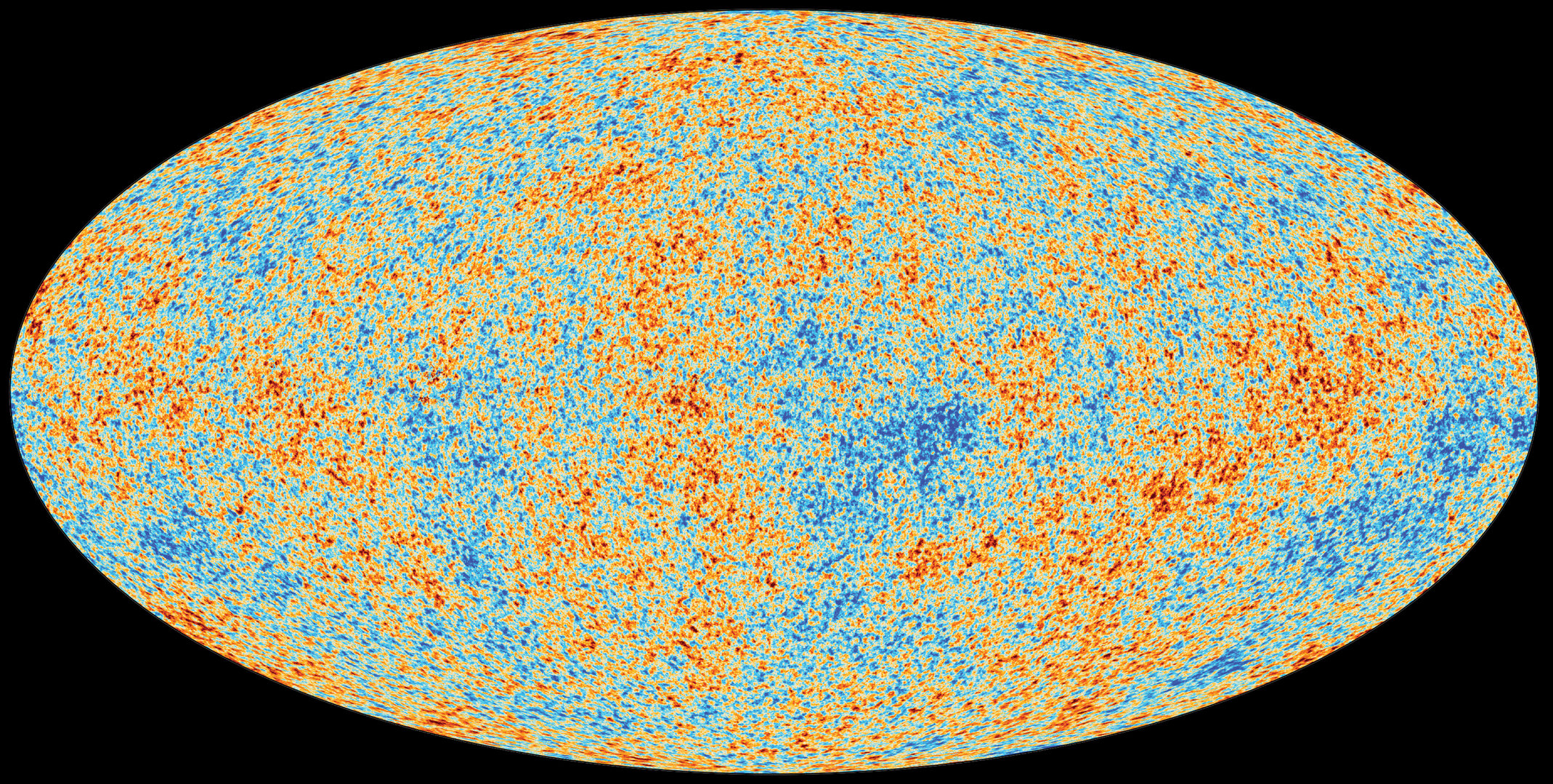
CMB: Quick Peak Estimate (Fast Math)
Using Wien’s law with \(T\approx 2.7\ \mathrm{K}\):
\[ \begin{aligned} \lambda_{\mathrm{peak}} &\approx \frac{0.29\ \text{cm}\cdot\text{K}}{2.7\ \text{K}} \\ &= \left(\frac{0.29}{2.7}\right)\ \text{cm} \\ &\approx 0.11\ \text{cm} \\ &= 1.1\ \text{mm} \end{aligned} \]
That’s about a millimeter → microwave.
A Subtle Plotting Warning (Optional)
The “peak wavelength” depends on whether you plot:
- \(B_\lambda\) (per wavelength interval), or
- \(B_\nu\) (per frequency interval)
Same physics; different axis choice can shift where the peak appears.
Part 2: Spectral Lines = Atomic Fingerprints
Continuum tells temperature; lines tell composition.
Why Lines Exist (One Sentence)
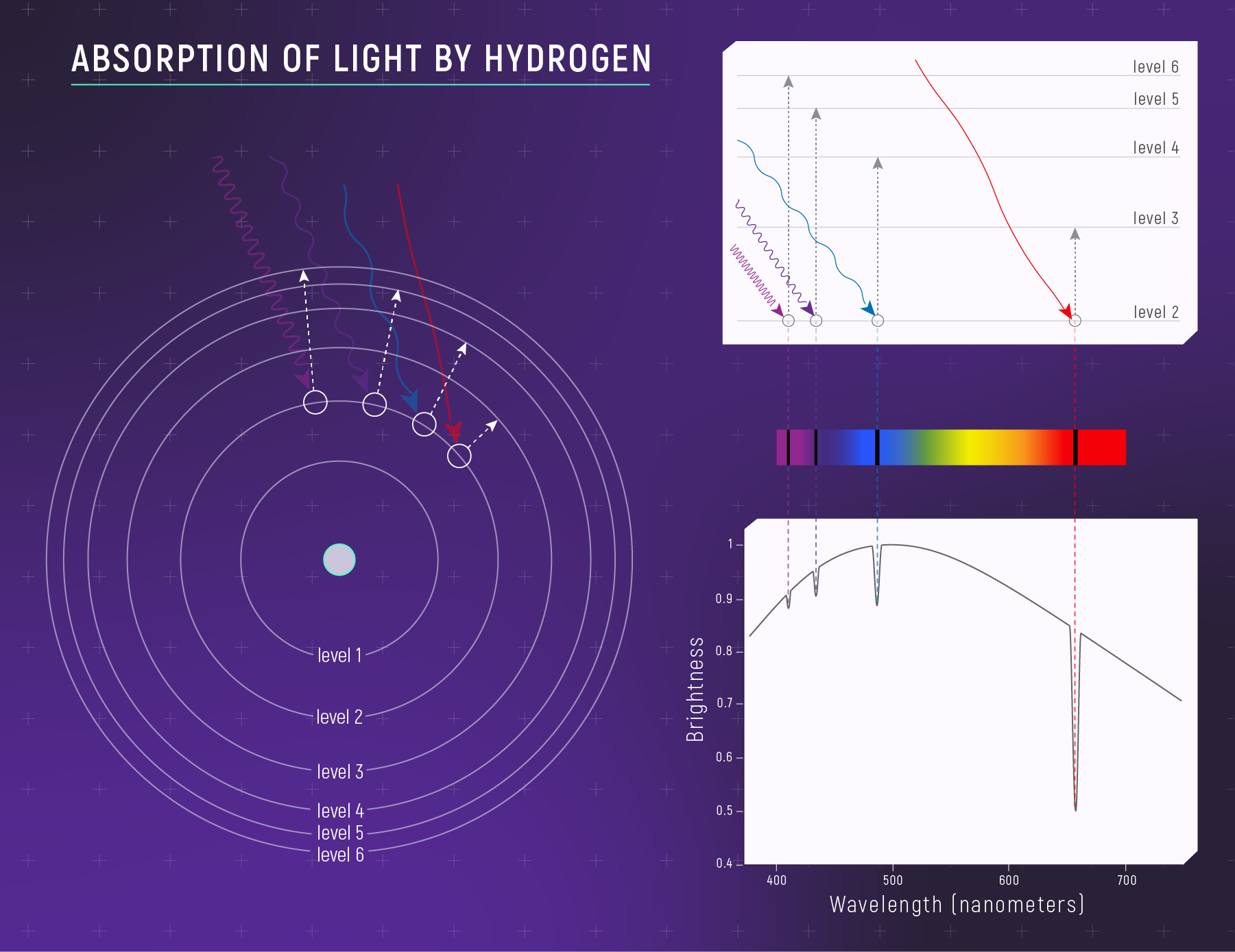
Atoms have quantized energy levels — they absorb or emit photons with specific energies.
The Bohr Model (A First Mental Picture)
Electrons live on “allowed steps” (energy levels).
- jump up → absorb a photon
- fall down → emit a photon
Absorption: Photon In, Electron Up
You see dark lines when:
- light passes through cooler gas
- specific photon energies are removed
Emission: Electron Down, Photon Out
You see bright lines when:
- excited gas emits
- photons come out at specific energies
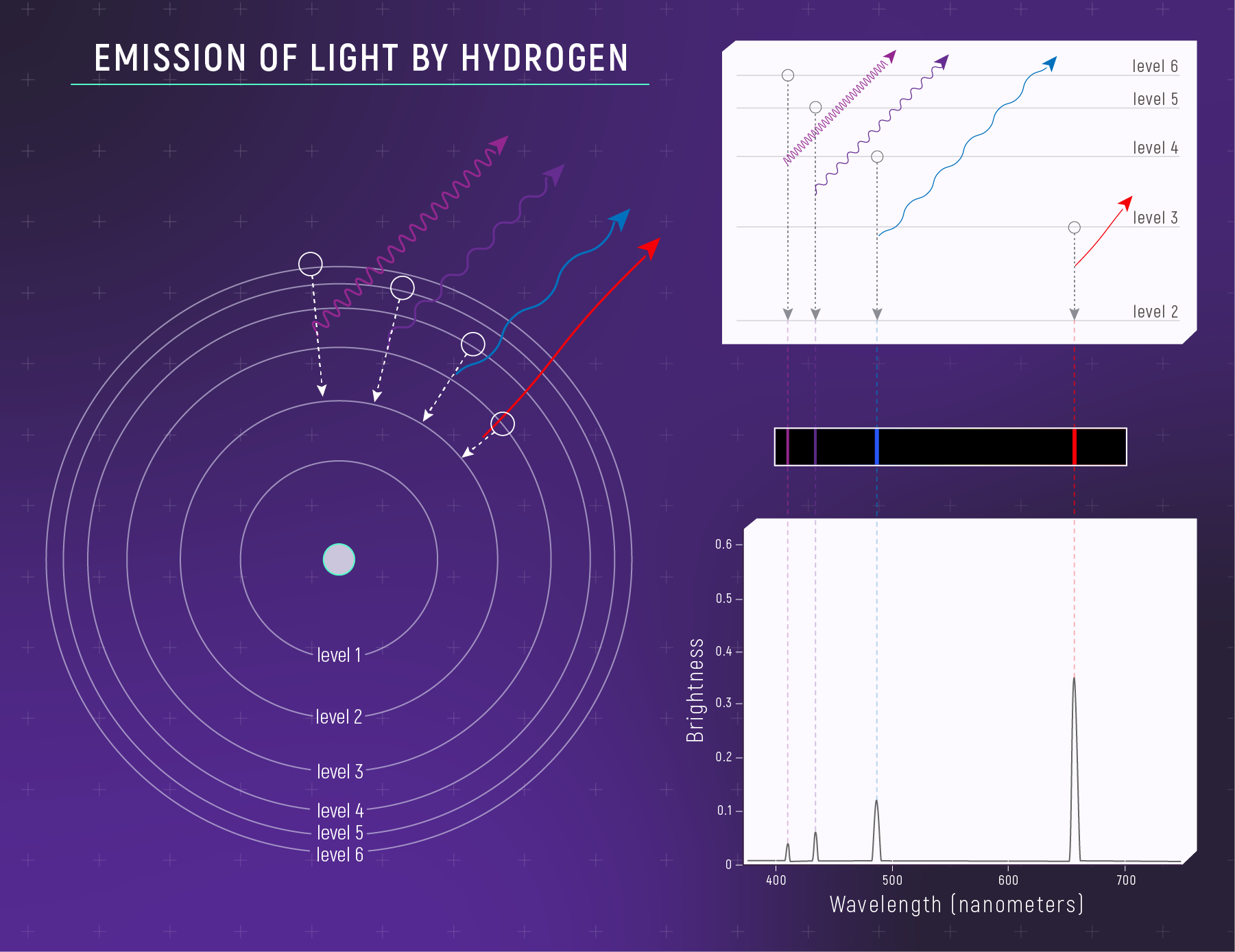
Kirchhoff’s Laws (The Three Spectrum Types)
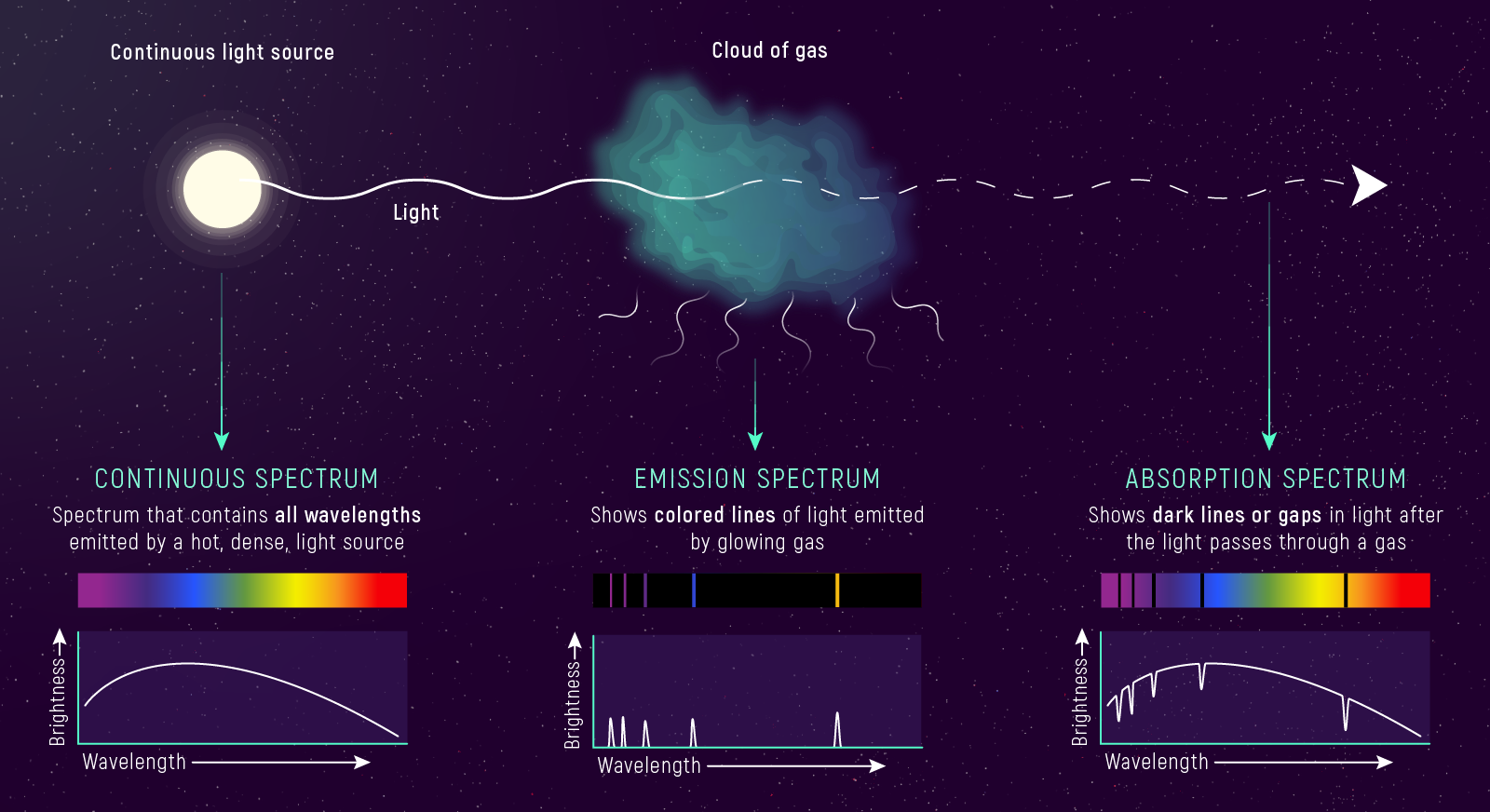
Kirchhoff Summary (For Studying)
- hot, dense → continuous
- continuous + cooler gas → absorption
- hot, thin gas → emission
🧠 Quiz: Which Spectrum?
A hot dense source shines through a cooler gas cloud in front of it. You observe…
- an emission-line spectrum
- an absorption-line spectrum
- a continuous spectrum with no lines
- a spectrum that depends only on distance
Quick Check: Name the Scenario
Which situation gives an emission-line spectrum?
- a hot dense object by itself
- a hot dense object seen through cooler gas
- a hot, low-density gas cloud
- a spectrum with no wavelength information
Element Fingerprints
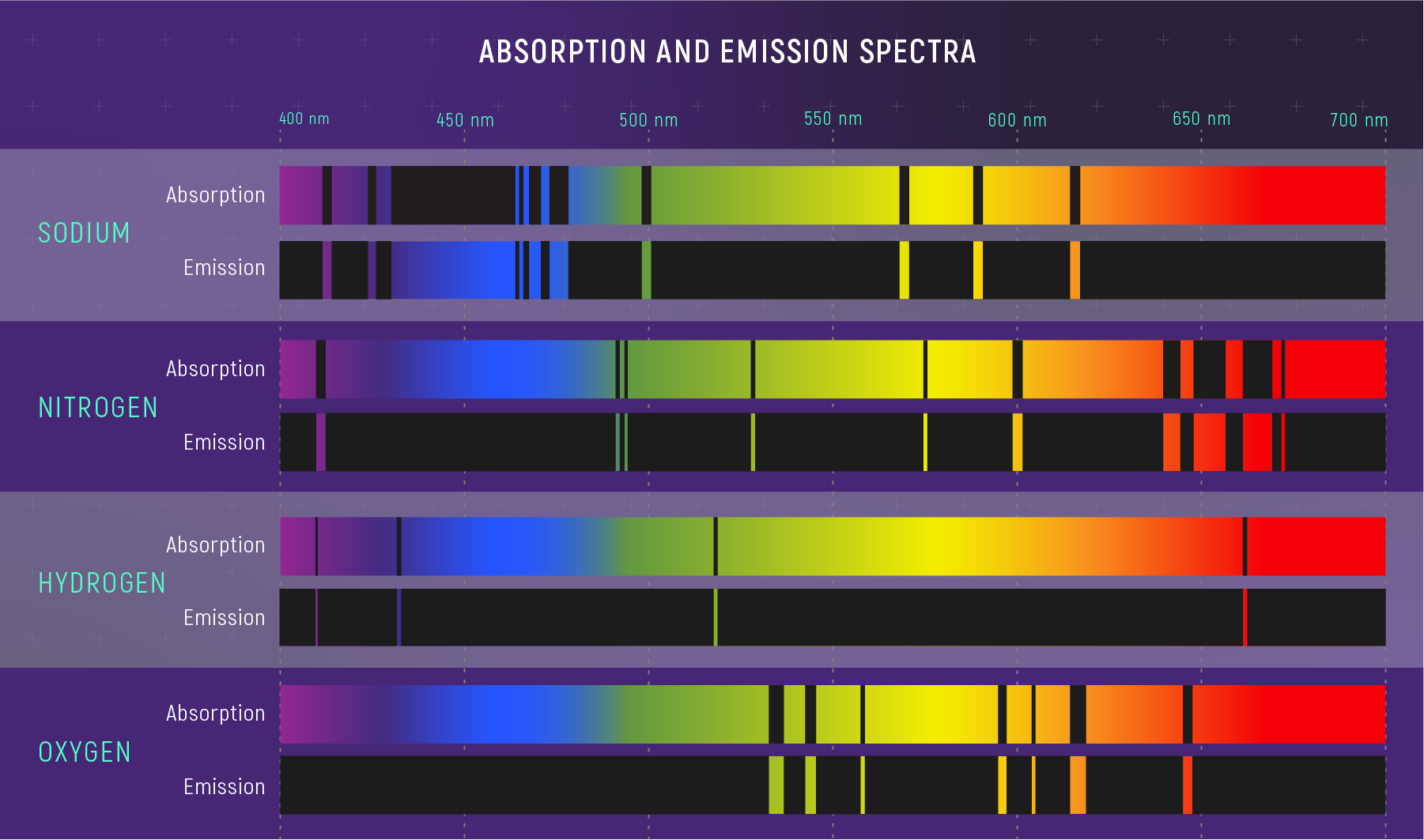
What Spectral Lines Can Tell You (Preview)
From lines alone, astronomers can infer:
- composition (which elements are present)
- temperature (which transitions are populated)
- density/pressure (line widths and ratios)
- motion (Doppler shifts — coming soon)
Real Stellar Spectra Combine Tools
In one observation, you can infer:
- temperature from the continuum shape
- composition from which lines appear
- (later) velocity from line shifts
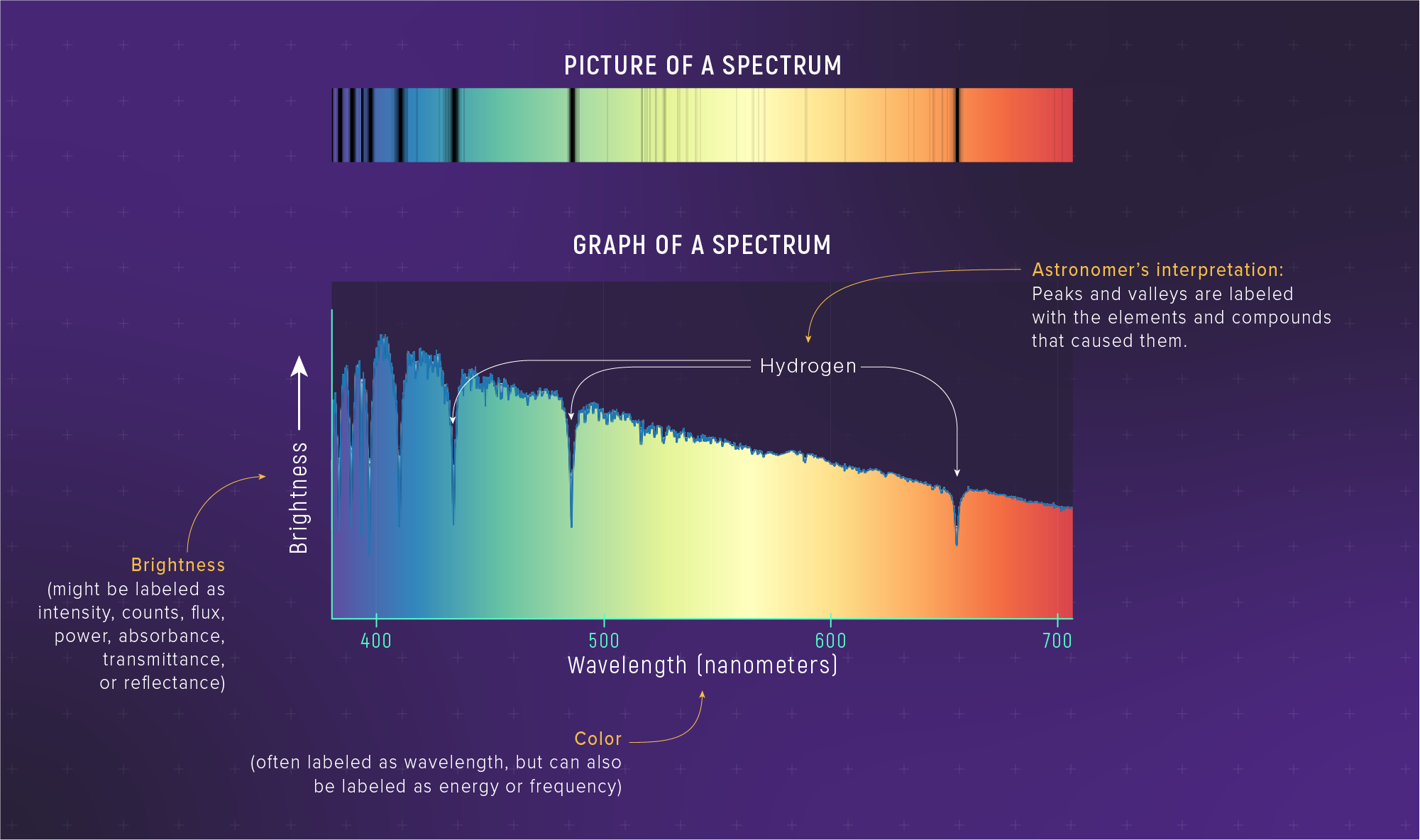
Thermal vs Non-Thermal Emitters
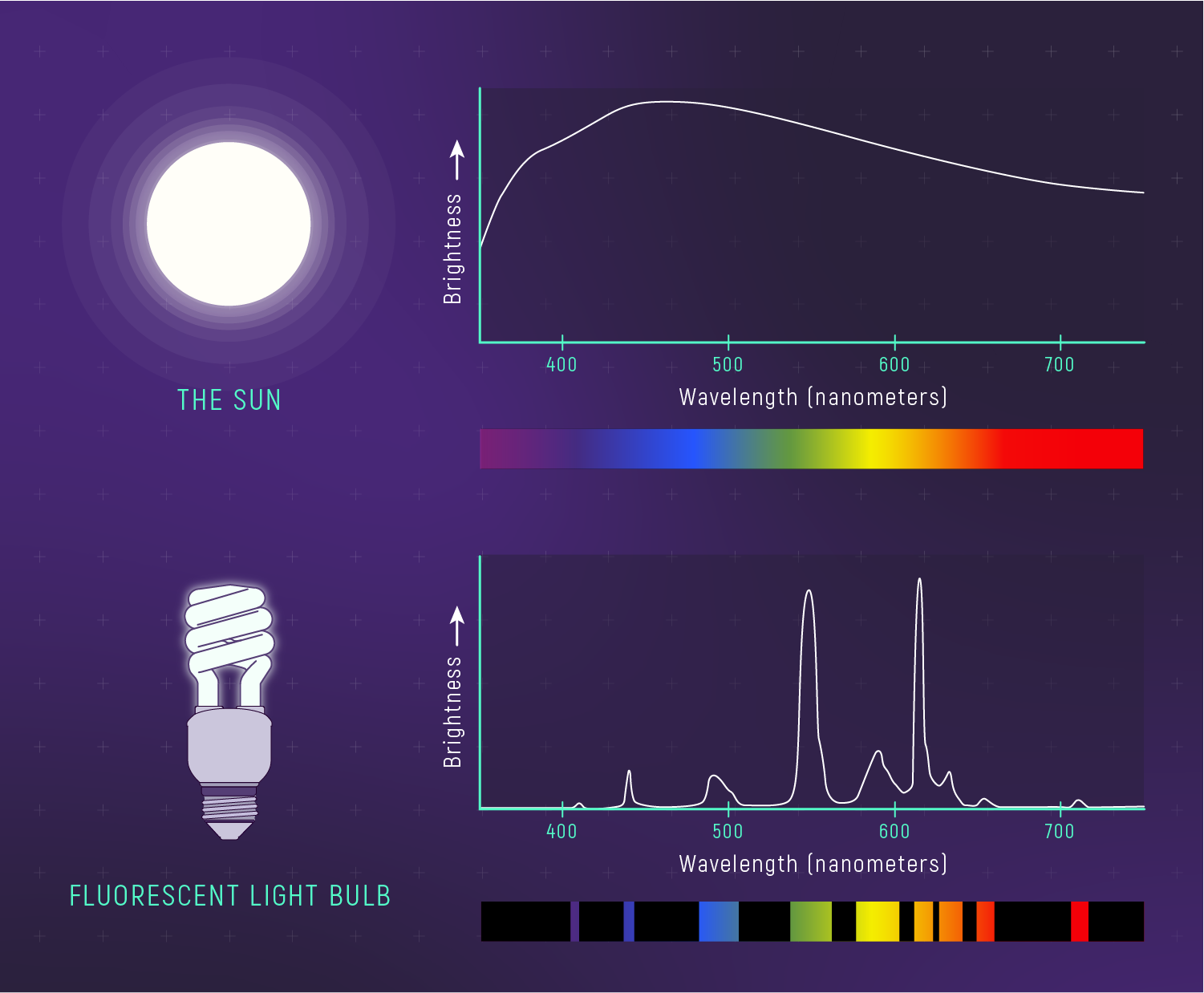
Doppler Shift = Motion from Light
Spectral lines are fixed fingerprints in the lab.
When those lines shift, we are seeing motion.
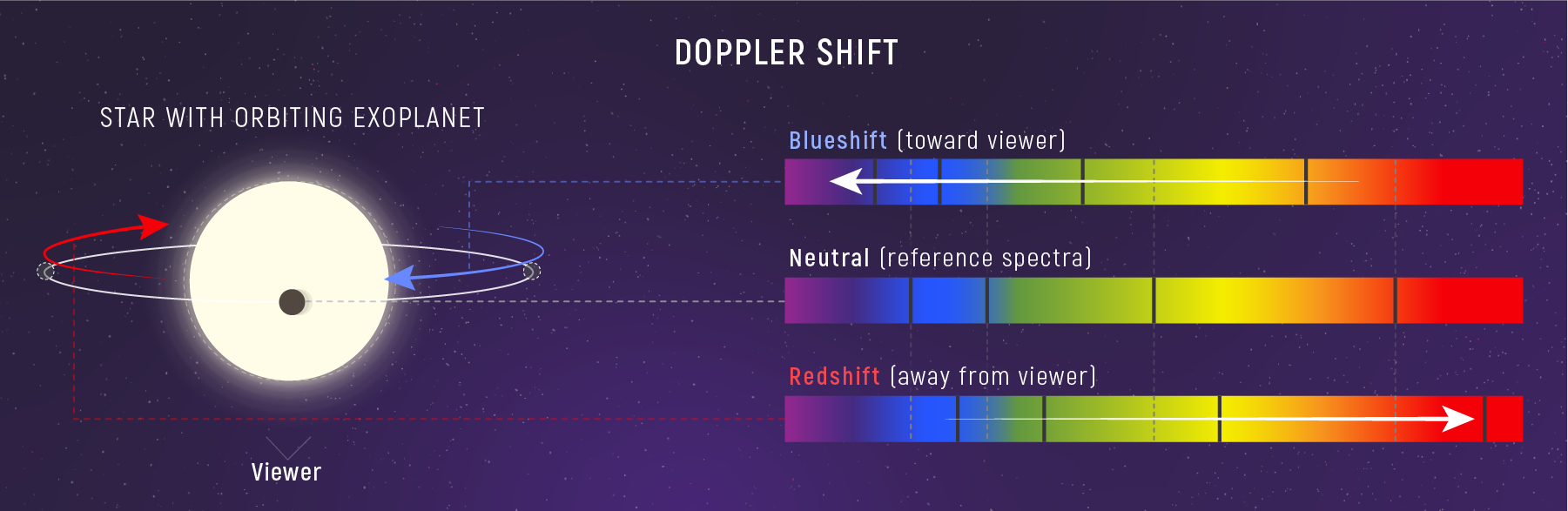
The Doppler Equation (Small‑Velocity Limit)
\[\frac{\Delta\lambda}{\lambda_0} = \frac{v_r}{c}\]
- \(\Delta\lambda = \lambda_{\text{obs}} - \lambda_0\) (shift from rest wavelength)
- \(v_r\) is line‑of‑sight velocity (positive = moving away)
- Assumes \(v_r \ll c\); if \(v_r = 0\), then \(\Delta\lambda = 0\)
Misconception Check: “Red” and “Blue”
Redshift does not mean “only red light.”
Blueshift does not mean “only blue light.”
- The entire spectrum shifts: every wavelength stretches or compresses.
- The names come from direction (toward red = longer \(\lambda\), toward blue = shorter \(\lambda\)), not from the color of the source.
Why This Is Powerful
Because of tiny line shifts, we can measure things we can’t touch:
- Exoplanets via stellar wobble
- Binary star masses from orbital speeds
- Galaxy rotation curves (dark matter)
- Cosmic expansion (redshift)
🧠 Quick Check: Direction
A spectral line appears at a longer wavelength than its lab value. The source is…
- moving away (redshift)
- moving toward (blueshift)
- motionless
- moving sideways only
Part 3: Telescopes Turn Light Into Inference
More photons and finer detail.
Collecting Area: Why Bigger Is Better
The number of photons you collect scales like:
\[A \propto D^2\]
Double \(D\) → 4× photons.

Collecting Area: Quick Example
If one telescope has diameter \(D\) and another has \(2D\):
\[\frac{A_2}{A_1} = \left(\frac{2D}{D}\right)^2 = 4\]
🧠 Predict (Collecting Area)
A telescope with 3× the diameter collects how much more light?
- 3×
- 6×
- 9×
- 27×
Angular Resolution: Why Bigger Is Sharper
Diffraction sets a fundamental limit:
\[\theta \propto \lambda D^{-1}\]
- bigger \(D\) → smaller \(\theta\) (sharper)
- longer \(\lambda\) → larger \(\theta\) (blurrier)

Misconception Check: Magnification vs Resolution
More magnification does not create more detail if the image is already blurred.
Resolution is set by \(D\) and \(\lambda\) (and the atmosphere), not the zoom knob.
The Rayleigh Criterion (Name the Rule)
For a circular aperture, the diffraction limit is approximately:
\[\theta \approx 1.22\,\lambda D^{-1}\]
🧠 Predict (Resolution)
Same telescope, same target. Switching from visible to infrared (longer \(\lambda\)) makes resolution…
- better
- worse
- unchanged
- dependent only on magnification
Instruments + Atmosphere
Two reasons we build space telescopes:
- blocked wavelengths (no ground X-ray astronomy)
- atmospheric blurring (seeing)
Mirrors vs Lenses (Brief)
Why big telescopes are mirrors:
- lenses absorb/warp light when they get large
- mirrors can be supported from behind
- reflective coatings can work across many wavelengths
Synthesis (Day 2)
We now have a powerful inference chain:
- continuum → temperature (blackbody + Wien)
- lines → composition (and motion)
- telescopes → more photons + better detail
Everything we do next semester-wide is built on this.
Demo Time (If Time): Telescope Resolution
Demo: telescope-resolution
Prompt: What matters more for resolution — aperture or magnification? What does wavelength do?
Exit Ticket (30 seconds)
Pick one:
- “One new inference we can make from spectra is…”
- “One reason we build big telescopes is…”
Study Snapshot (Write This Down)
- continuum → \(T\) (blackbody + Wien)
- lines → composition
- \(A\propto D^2\) (photons)
- \(\theta\propto \lambda D^{-1}\) (detail)

ASTR 201 • Dr. Anna Rosen • Lecture 4 (Day 2)